






| General Formulas | |
| Density | 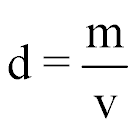 |
| Moles | 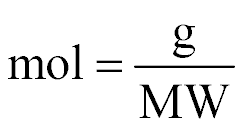 |
| Equivalents | 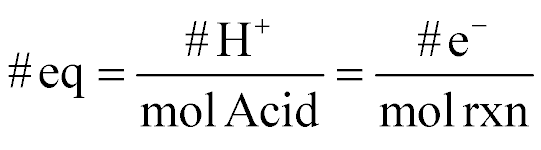 |
| Converting from °F to °C | 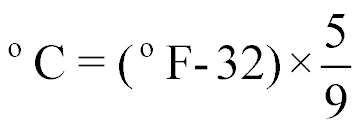 |
| Converting from °C to °F | 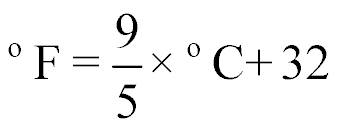 |
| Converting from °C to K | |
| Percent Yield | 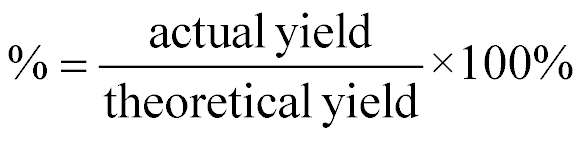 |
| Electrical Force between 2 charges | 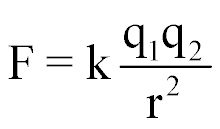 |
| Kinetic Energy | 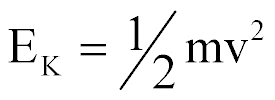 |
| Solution Formulas | |
| Molarity | 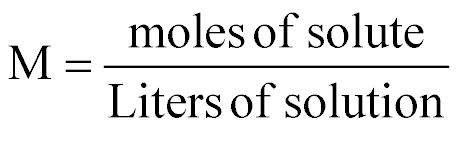 |
| Molality | 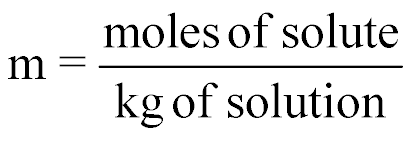 |
| Normality | 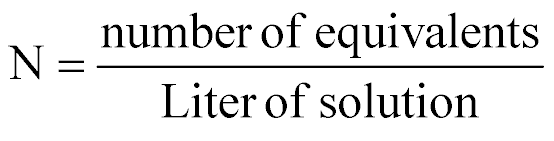 |
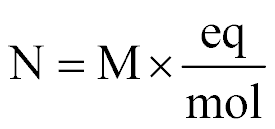 | |
| Mole Fraction | 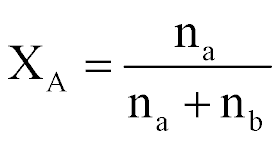 |
| Dilution | |
| Solubility Product Const (Ksp) | |
| Definition of pKsp | |
| Gases and Gas Laws | |
| Boyle's Law of Gases | |
| Charles' Law of Gases | 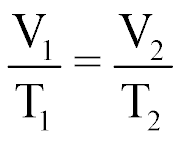 |
| Gay-Lussac's Law of Gases | 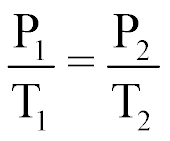 |
| Combined Gas Law | 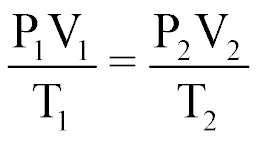 |
| Ideal Gas Law | |
| Density or Molar Mass of Gas | 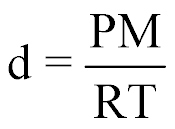 |
| Dalton's Law of Partial Pressures | |
| Root Mean Square Speed of a Gas | 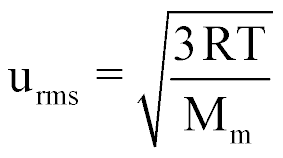 |
| Van Der Waal's Equation | 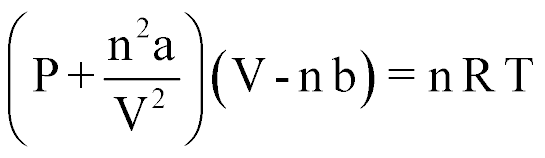 |
| Graham's Law of Effusion' | 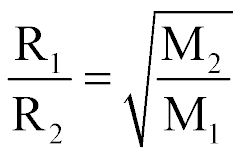 |
| Henry's Law | |
| Thermochemical Formulas | |
| Specific Heat | |
| Enthalpy Change of Reaction | |
| First Law of Thermodynamics | |
| Work Done in Gas Expand/Compress | |
| Definition of Enthalpy | |
| Enthalpy Change (Const P) | |
| Clasius-Clapeyron Equation | 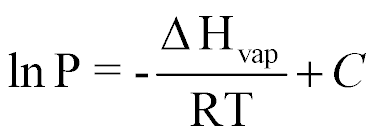 |
| Calculation of Hvap, vp, or bp of a liquid | 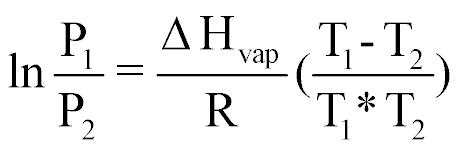 |
| Raoultís Law | |
| Boiling Point Elevation | |
| Freezing Point Depression | |
| Osmotic Pressure of a Solution | |
| Gibbs Free Energy Change | |
| Standard Entropy Change | |
| Standard Free-Energy Change of a Reaction: | |
| Free-Energy Change and Reaction Quotient | |
| Standard Free-Energy Change and the Equilibrium Constant | |
| Equilibrium Formulas | |
| Rate Law Expression | |
| Concentration and Time for a 1st-order Reaction | 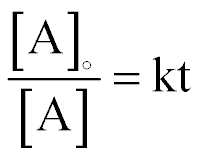 |
| Graphical Determination of K for a 1st-Order Reaction | |
| Half-life for a 1st-order Reaction | 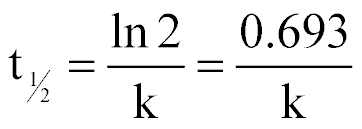 |
| Concentration and Time for a 2nd-order Reaction | 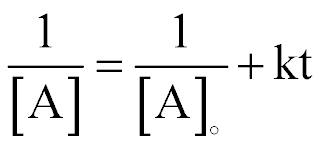 |
| Arrhenius Equation | 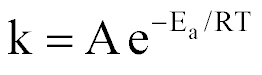 |
| Graphical Determination of Activation Energy | 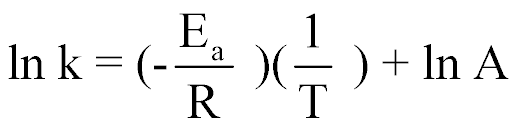 |
| Rate Constants at Two Different Temperatures | 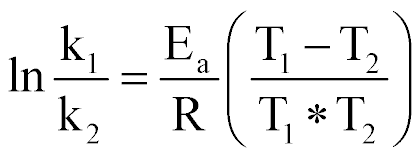 |
| General Expression of Equilibrium Constant: | 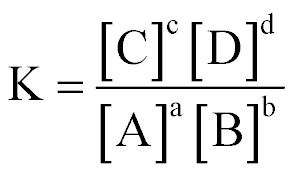 |
| Acids and Bases | |
| Ion-product Constant of Water: | |
| Acid Equilibrium Const (Ka) | 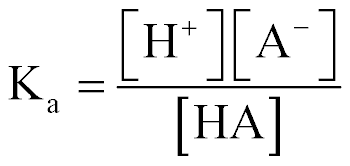 |
| Base Equilibrium Const (Kb) | 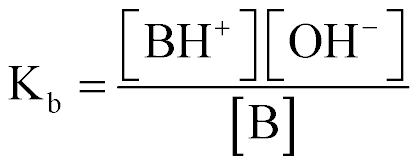 |
| Definition of pKa | |
| Definition of pKb | |
| Percent Dissociation | 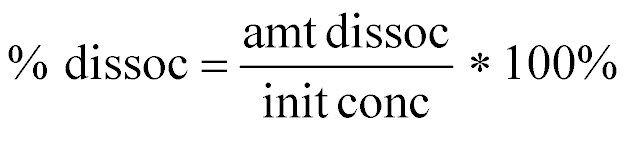 |
| Definition of pH of a Solution: | |
| Definition of pOH of a Solution | |
| Ionization Const of a Conj Acid-Base | |
| Henderson-Hasselbach Equation | 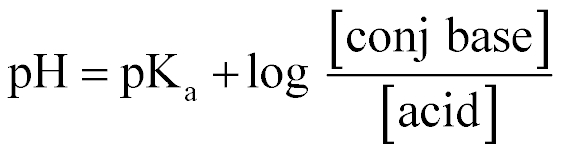 |
| Alt Relationship Between pH and pOH | |
| Atomic Chemistry | |
| Wavelength Frequency Relationship | |
| Energy of Photon | |
| Energy of an e- in the nth state in a Hydrogen atom | 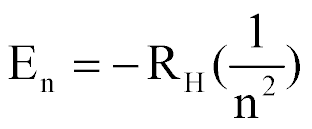 |
| Energy emitted as the e- transitions from the nilevel to the nf level | 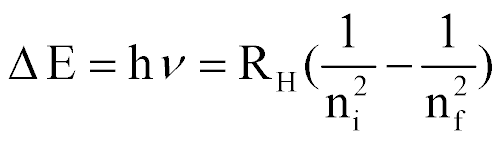 |
| Mass Defect and Energy Released | 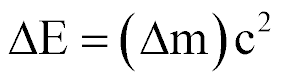 |
| Bragg Equation | |
| Electrochemistry | |
| Standard EMF of an Electrochemical Cell | 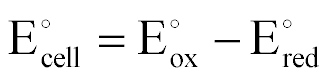 |
| Standard Free Energy Change | 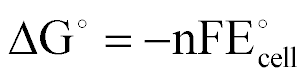 |
| Std EMF of the Cell to the Equilibrium Constant | 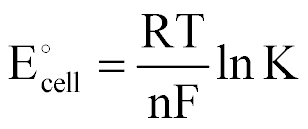 |
| The Nernst Equation | 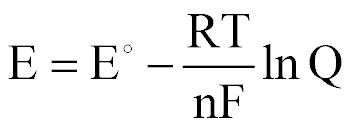 |